The definition of a drug, from the U.S. Food and Drug Administration (FDA), may be a product that’s identical, or bioequivalent, to a name drug in dose kind, safety, strength, route of administration, quality, performance characteristics and supposed use. Generic medicine square measure with chemicals just like their branded counterparts, howeverthey’re generally oversubscribed at well lower costs. in line with the legislative assembly Budget workplace, generic medicine save customers Associate in Nursing calculable $8 to $10 billion a year at retail pharmacies. Billions a lot of square measure saved once hospitals use generics.
Generic medicine should pass rigorous controls, rather like the innovative drug originally created. there’s no loss in quality, strength, purity and stability of the drug. However, patients might have the perception that a drug isn’t as effective because the original name.

| So, let’s take a look at some facts and evaluate what is known about generic drugs as well as brand name drugs. |
| The development of a new drug is a very complex process, which involves not just the research and development of the chemical, but also clinical trials and all of the regulatory processes to get the drug approved for human use. The cost of this process has been estimated to be as much as $800 million. Such an investment needs to be recovered and there needs to be sufficient margin for pharmaceutical companies to re-invest in more research and development, and keep the wheels rolling in biomedical applied research.
|
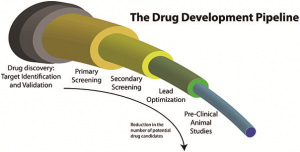
However, there is no doubt that alternatives to these high cost therapies are needed, regardless of how much effort or investment goes into developing a drug. Some countries or individuals simply cannot afford to pay such a high price. Thus, since the approval of the Drug Price Competition and Patent Term Restoration Act in 1984 (USA), generics have opened the doors to those with less resources. It is also helping to reduce health care costs in developed countries.

Now from the FDA perspective, here are some important facts that support the use of generics:
FDA requires generic drugs to have the same quality and performance as brand name drugs.
Research shows that generics work just as well as brand name drugs.
When it comes to price, there is a big difference between generic and brand name drugs. On average, the cost of a generic drug is 80% to 85% lower than the brand name product.
Cheaper does not mean lower quality.
FDA closely monitors adverse events reports for generic drugs.
FDA is actively engaged in making all regulated products – including generic drugs – safer.
However, it’s a difficult task for watchdogs just like the office to ensure the performance of the generic medicine. therefore let’s verify the opposite facet. In different words, ar generics really a twin of the whole drugs?
The office needs that you just get between eightieth to one hundred and twenty fifth of the brand drug activity from a generic medication, compared to the first drug. there’s additional variability additional if you get your generic from totally different vendors, together might pack eightieth of “effective” drug and another one a hundred and twentieth, for instance.
Components aside from active ingredients don’t seem to be needed to be identical and therefore the formulation maydoubtless have an effect on however the drug is delivered to your blood stream.
Regarding the controls, the office admitted that some generics had not been tested, and even force one out from the market.

So what can you do if you feel your body is not responding in the same way to the brand name drug and the generic ones?
Find out if an “authorized” generic exists for your drug. These are generics typically made by the same manufacturer of the brand name medication but sold under a generic brand name. They are not similar, but identical. Ask your pharmacist if one exists for your medication.
When switching to a generic, monitor your condition carefully. When switching from a brand name to a generic drug, or from one generic to another, note any changes you feel and tell your doctor immediately.
Finally, and more importantly, always consult your doctor regarding any questions you have regarding generic and brand name drugs. Always make an informed decision with the help of your health care provider. The points expressed in this post are just factual and for the only purpose of promoting a healthy debate (with the emphasis on healthy when talking about drugs).

| If you want to know more, you can take a look at these websites:
Generic Drugs: Questions and Answers from the FDA |
